The methods used by various industries to achieve carbon reduction targets can be summarized as follows: (1) CO2 emission reduction; (2) Absorption/conversion of CO2.Converting CO2 into carbon electrode materials is a potential opportunity for the new energy industry,Carbon materials have been widely used as electrode materials for various energy storage devices, such as lithium batteries and supercapacitors.CO2 can be converted into a variety of high value-added products, but only if the conversion process is green, zero emission and low cost, otherwise it runs counter to the goal of carbon neutrality.Therefore, the conversion of CO2 into carbon electrode materials must also meet this premise.However, the most commonly used activated carbon materials need to be prepared by "carbonization - activation - purification" process, it not only does not consume CO2, but also emits a lot of CO2 and waste liquid. The progress based on CO2 synthesis of carbon materials shows that no matter using LiALH4 to regenerate or using Mg with CO2 to make direct reaction, the carbon materials have a low surface area, which is not conducive to increase the capacity of supercapacitors.However, the template carbon synthesized by traditional non-cyclic MgO template method has high specific surface area and high conductivity, the two traits are very suitable for supercapacitors, it uses benzene with high toxicity as the carbon source and HCl to remove template,the template can not be recycled ,as a result,the raw material and environmental costs of template carbon synthesis are high and hinder its large-scale application.
Recently, the School of Mechanical Engineering/Institute of Advanced Manufacturing and Modern Equipment Technology Engineering of Jiangsu University published a paper entitled "Removing Cost Barriers to Template Carbon Synthesis for High-Performance Supercapacitors by Establishing a Zero Emission Chemical Cycle from CO2 "(ACS Energy Lett. 2022, 7, 4381-4388) in the internationally renowned journal ACS Energy Letters (SCI Section I, Impact factor 23.991).This paper based on CO2 constructs a zero-emission chemical cycle, realizing the recycling of MgO template (including its dissolution and regeneration) and the green synthesis of template carbon with high specific surface area, and effectively solving the cost and emission problems of template carbon (TC) synthesis.Capacitors assembled with symmetrical template carbon electrodes show excellent capacitance and multiplier performance in aqueous (KOH/H2O, >154 F g-1) or organic (Et4NBF4/AN, >140 F g-1) and ionic liquid (EMIMBF4, >178 F g-1) electrolyte. The maximum energy density of the device is 17 Wh kg−1, which is significantly higher than that of the traditional supercapacitor (< 10 Wh kg-1). Both in the water system and in organic/ionic liquids, it also shows good cyclic stability, and the capacitance retention rate is higher than 90.5% after 20000 times of charge and discharge.
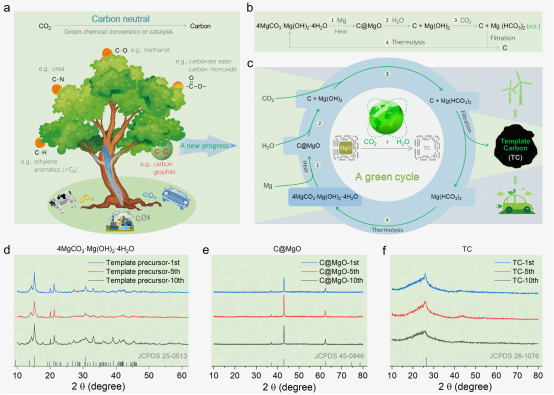
The paper is signed the School of Mechanical Engineering/Institute of Advanced Manufacturing and Modern Equipment Technology Engineering of Jiangsu University as the first unit, with Liang Hongyu as the first author and Bu Yongfeng from the Institute of Energy Research as the corresponding author. This research was supported by the National Natural Science Foundation of China (52075224, 21975109). In addition, the team has published their work on electrolyte additives in Nature Index Chemical Communications 2022, 58(68), 9536-9539. See the link below for details.
Link to the article:
https://pubs.acs.org/doi/10.1021/acsenergylett.2c02203
https://pubs.rsc.org/en/content/articlelanding/2022/cc/d2cc03732g
